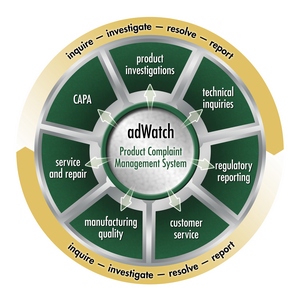
adWATCH - Product Complaint Management (PCM) helps organizations capture, distribute, retrieve, and manage product complaints globally and in real-time.
The objective of Atlant Systems’ adWATCH-PCM Product Complaint Management system is to help organizations capture, distribute, retrieve, and manage product complaints globally -- product complaints from any manual source, computer source or application. adWATCH-PCM fosters an environment where document creation, review, and approval can be done collaboratively among individuals within and outside the corporation all over the world.
adWATCH-PCM helps a pharmaceutical and/or medical device company:
- Receive, review and evaluate product complaints from customers, physicians, patients, distributors, and other organizations
- Process and document investigations of product complaints by the respective manufacturing site(s)
- Resolve product complaints including the actions necessary to meet customer service goals
- Track all open product complaints to ensure timely resolution and action
- Enforce an approval process for each product complaint that complies both with company and regulatory requirements
- Provide access to the product complaint management system by all authorized users inside and outside the organization
- Archive product complaint documents electronically for rapid search and retrieval
- Deliver ad hoc reporting to management for trend evaluation and statistical analysis
Features and Benefits
- adWATCH - PCM automatically generates the FDA-approved MedWatch, Baseline, and CIOMS reports from information already in the database.
- adWATCH - PCM allows remote users to participate in many functions of the system via dial-up, intranet, or extranet.
- adWATCH – PCM mirrors the workflow of complaint processing from initial notification through investigation, analysis, and resolution.
- The Customer Notification forms letter library provides the ability to send customized letters at predetermined times or on an as-needed basis during the investigation and resolution.
- adWATCH - PCM provides the ability to respond quickly to product quality issues by providing timely customer feedback.
- adWATCH - PCM includes a software validation database to assist with FDA-mandated requirements for initial validation and change control procedures.
- Enterprise-wide replication ensures up-to-the-minute information is available across all locations, worldwide
- An intuitive look and feel to screens makes using adWATCH - PCM simple and easy to use.
Download Brochure, Click here
